How Many Elements Are In Zn

For instance francium results from decayed actinium.
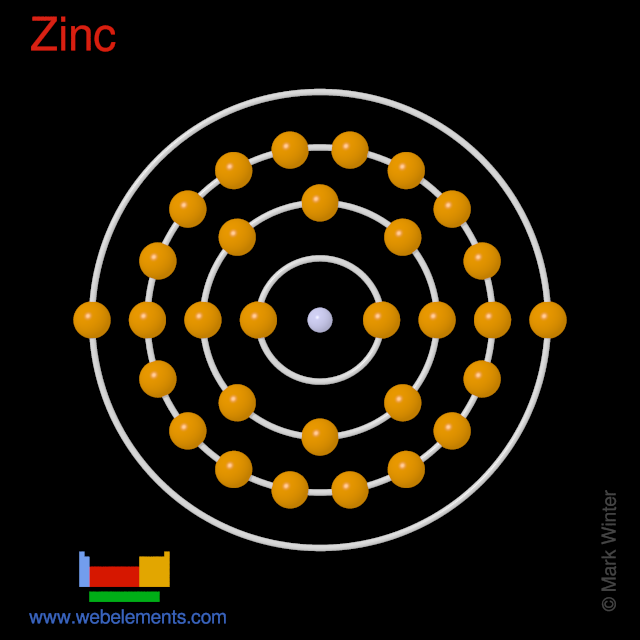
How many elements are in zn. 5 1 2 3 4 z 4. The more stable cubic form is known also as zinc blende or sphalerite the hexagonal form is known as the mineral wurtzite although it also can be produced synthetically. Zinc hydroxide contain 3 elements. It is the first element in group 12 of the periodic table in some respects zinc is chemically similar to magnesium.
Rare vs native elements. Note that 3 3 1 mod 4. Noncombustible but accelerates the burning of combustible materials. Zinc is a slightly brittle metal at room temperature and has a blue silvery appearance when oxidation is removed.
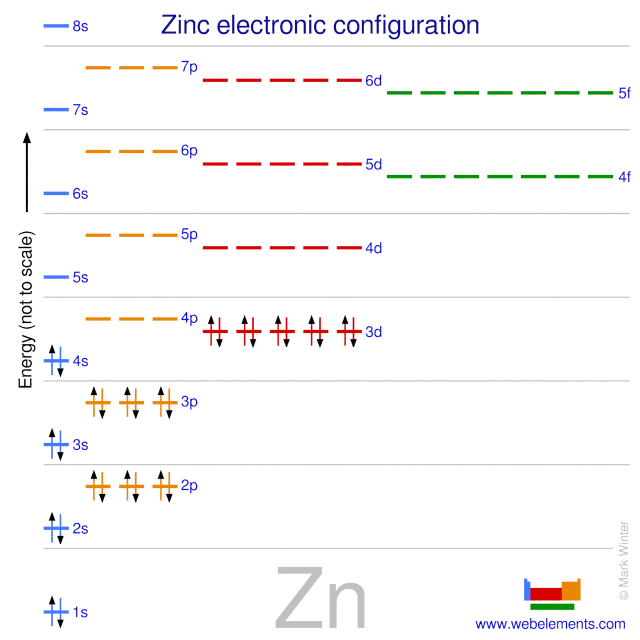
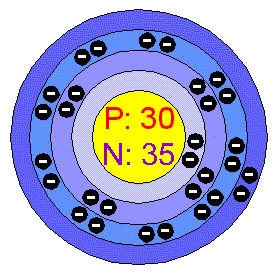
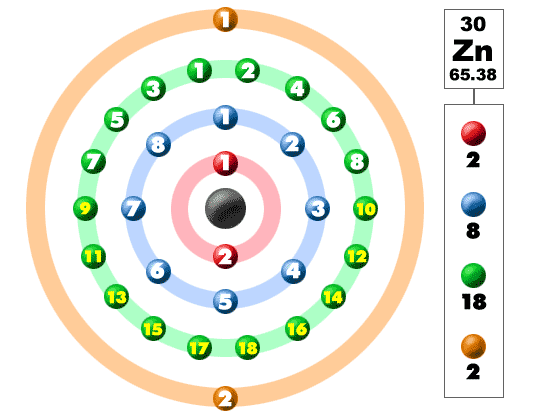
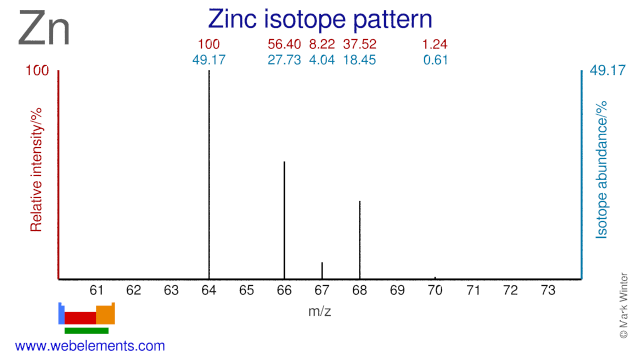
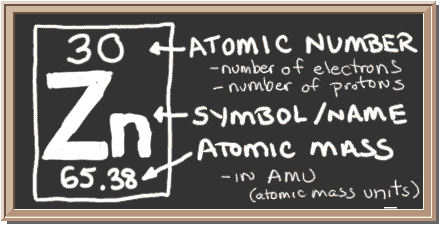
The one element group is unique up to isomorphism. Zinc is a chemical element with the symbol zn and atomic number 30. The transition from the sphalerite form to the wurtzite. Over 48 6 of naturally occurring zinc is in the form of 64 zn.
Represented in the periodic table as zn zinc is a transition metal grouped with cadmium and mercury. Zinc group element any of the four chemical elements that constitute group 12 iib of the periodic table namely zinc zn cadmium cd mercury hg and copernicium cn. 4 1 3 z 2. Note that 2 2 4 2 3 3 and 2 4 1 mod 5.
Note that 5 5 1 mod 6. A number of the elements listed in the periodic table recently may have been produced through the decay of unknown elements that have been in existence for a long time. With the middling atomic number 30 it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70 plus an extra 25 radioisotopes. Rare elements are obtained through the radioactive decay of some common elements.
Naturally occurring zinc is a mixture of five isotopes and they are found in the percentages shown. 64 zn 48 6 66 zn 27 9 67 zn 4 1 68 zn 18 8 and 70 zn 0 6. Stack exchange network consists of 177 q a communities including stack overflow the largest most trusted online community for developers to learn share their knowledge and build their careers. Both elements exhibit only one normal oxidation state 2 and the zn 2 and mg 2 ions are of.
When p is prime the units in z p always form a cyclic group of order p 1. 6 1 5 z 2. If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. See remark above at m 3.
Also there is a unique group of order 2. They have properties in common but they also differ in significant respects.










/GettyImages-172430480-5c54686f46e0fb00013a202d.jpg)