How Many Elements Are In Group 1
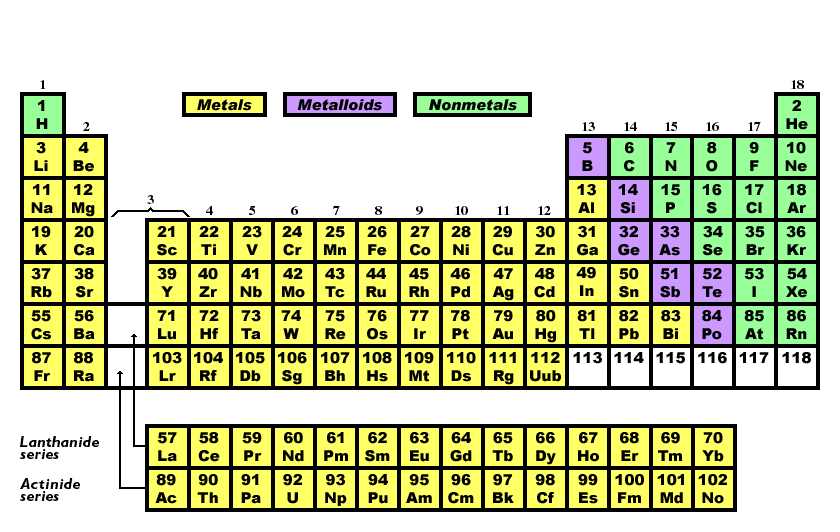
There are 7 elements in group 1 in periodic table.
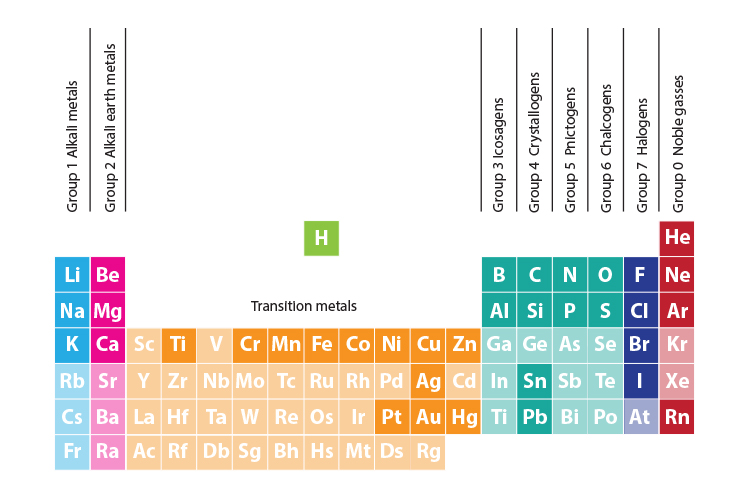
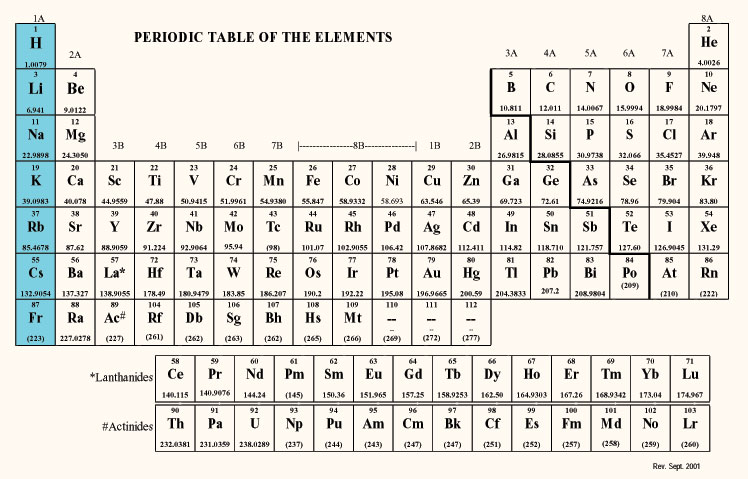
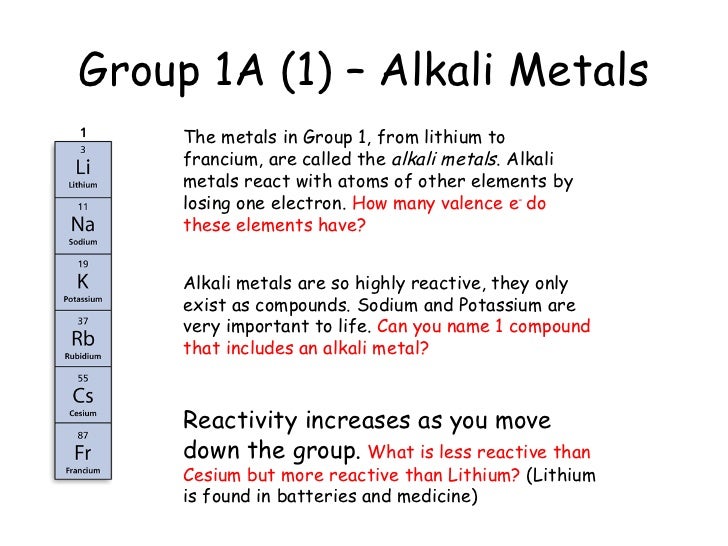
How many elements are in group 1. Group 1 is on the left hand side of the. Placed in a vertical column on the far left of the periodic table. Alkali metals are the elements of group 1 of the periodic table that when reacts with water produces an alkaline solution along with the release of hydrogen gas. The reactivity of the alkali metals increases down the group.
All these elements have just one electron in the very outside layer of the electrons that surround the nucleus. The group 1 elements in the periodic table are known as the alkali metals. Hydrogen is not considered to be an alkali metal as it rarely exhibits behaviour comparable to theirs though it is more analogous to them than any other group. Alkali metals include lithium sodium potassium rubidium and cesium.
The elements in group 1 are often called the alkali metals. There are six elements in group 1 of the periodic table and they are. They include lithium sodium and potassium which all react vigorously with air and water. Elements of the group have one s electron in the outer electron shell.
You can see them in the first column of the periodic table below.