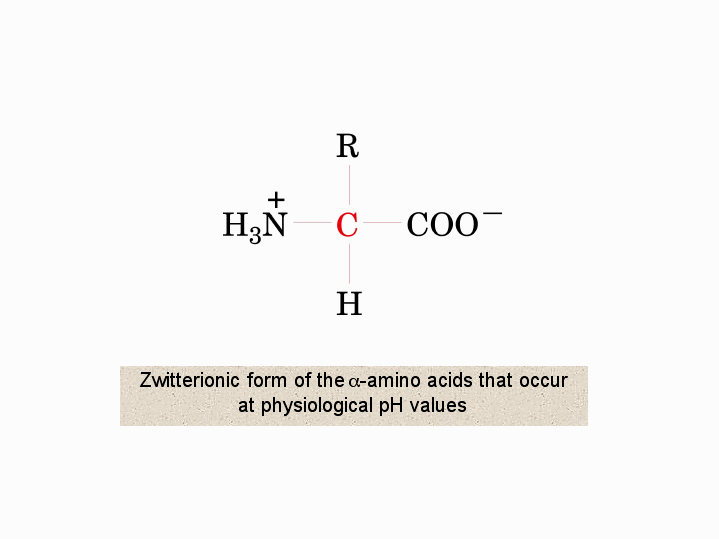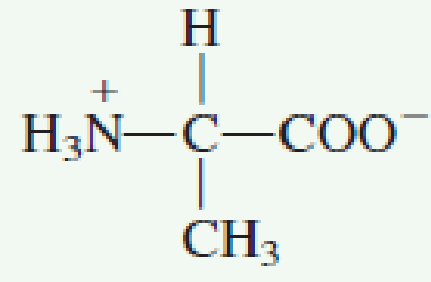How Many Amino Acids Are Zwitterions
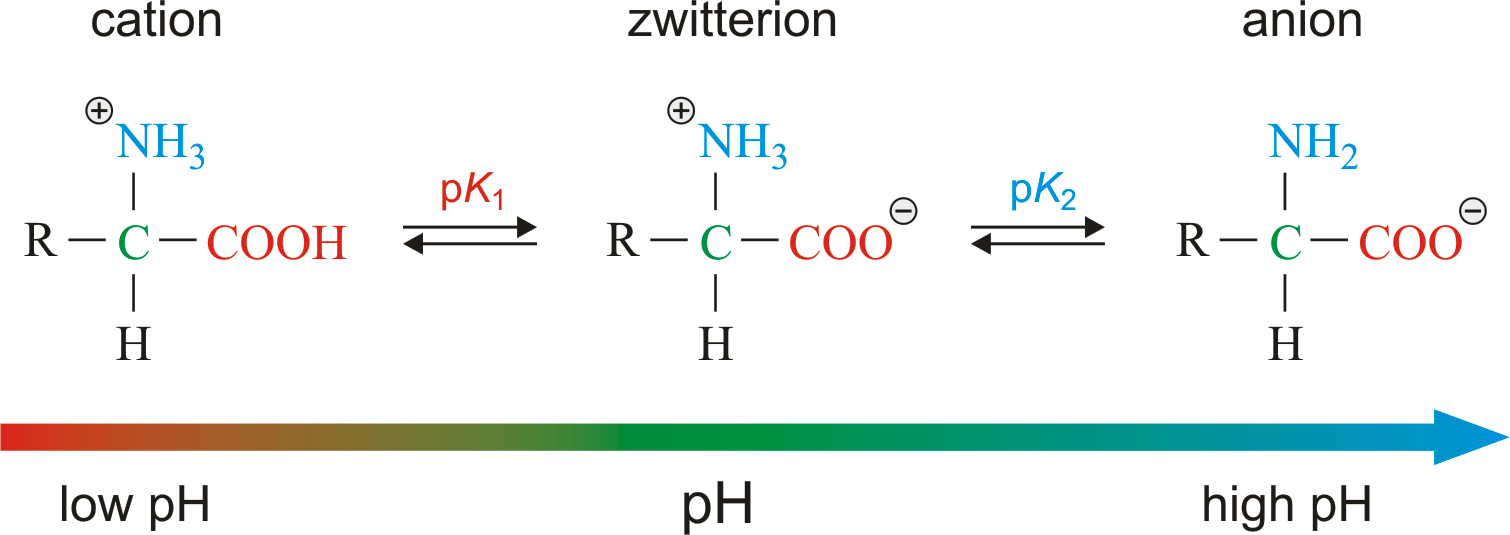
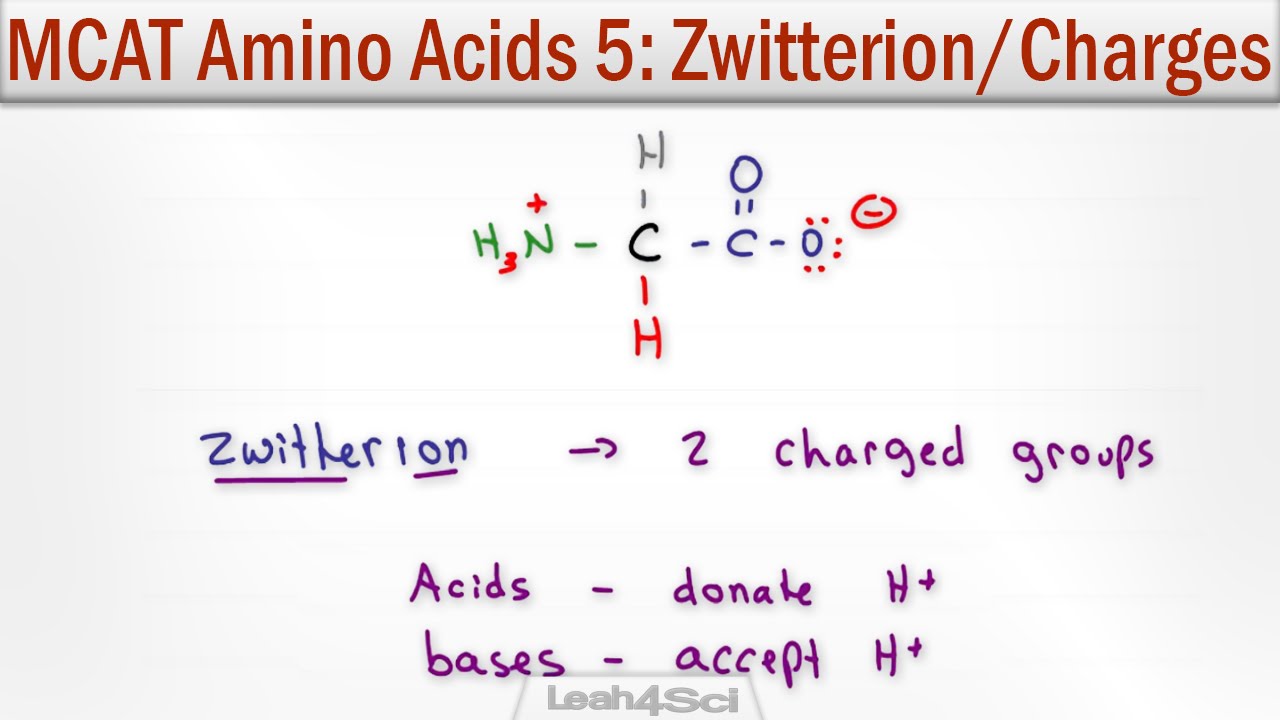
Zwitterions and amino acids a zwitterion is a molecule with functional groups of which at least one has a positive and one has a negative electrical charge.
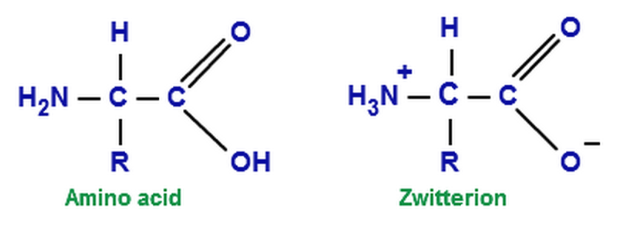
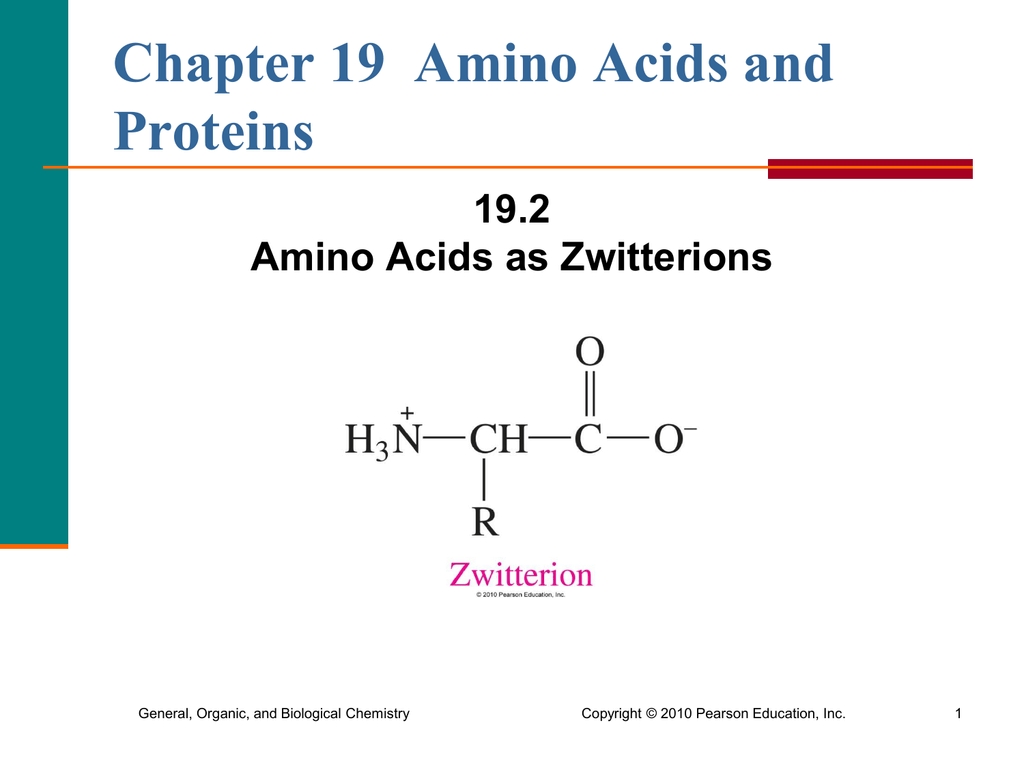
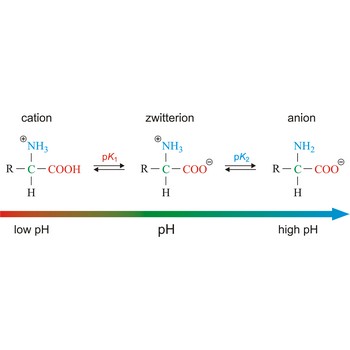
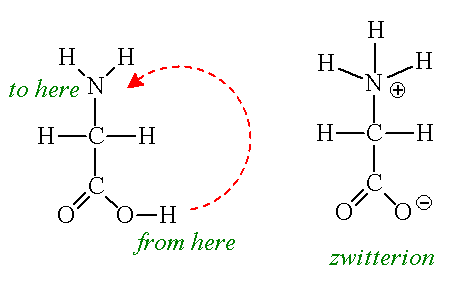
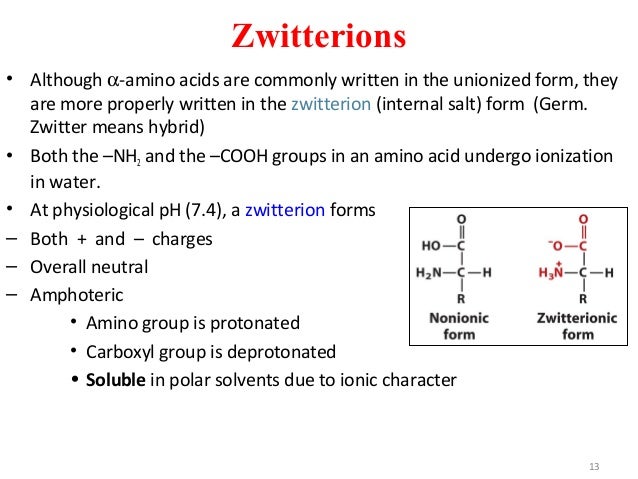
How many amino acids are zwitterions. There is an internal transfer of a hydrogen ion from the cooh group to the nh 2 group to leave an ion with both a negative charge and a positive charge. Zwitterions in simple amino acid solutions. The pi or isoelectric point corresponding to the zwitterion form lets you calculate the ph at which an amino acid will have a net zero charge. An amino acid has both a basic amine group and an acidic carboxylic acid group.
The net charge of the entire molecule is zero. The isomer on the right is a zwitterion. Amino acids are the best known examples of zwitterions. The equilibrium is established in two stages.
Amino acids can exist as zwitterions containing a protonated amine group and deprotonated carboxyl group. The net charge of the entire molecule is zero. At physiological ph monoaminomonocarboxylic amino acids e g glycine and alanine exist as zwitterions. Amino acids are the best known examples of.
They contain an amine group basic and a carboxylic group acidic. An amino acid contains both acidic carboxylic acid fragment and basic amine fragment centres. A zwitterion is a molecule with functional groups of which at least one has a positive and one has a negative electrical charge. The isomer on the right is a zwitterion.